What is Energy? Energy is a fundamental constituent of our universe. Sometimes it’s easier to use examples. For instance, we say a car has lots of energy if it is moving fast with respect to a road. We say molecules are very energetic when they vibrate due to ‘heat’. We say pulsars emit energy as they blast cosmic rays out from their core. Energy equates with ‘something doing something’.
We also say that energy is neither created or destroyed. There is the famous equation relating energy to mass. But this is a transfer of energy rather than a loss or gain. Energy’s conservation property makes it very suitable as a metric with which to assess civilizations’ past and future.
Let’s explore a bit more in the following.
Energy
The word energy gets readily bandied about. In this website, it’s meaning is reserved to be a fundamental unit of measurement. With this, energy is directly related to mass as in;
| Eq. 1 | Energy = Mass * (speed of light)2 |
| – or – | E = m c2 |
Don’t worry about this equation; we won’t be using it. But it is rather cool that energy has been shown mathematically to be so fundamental to our existence.
There is a choice of units for energy. This website uses the Joule. This unit name is in honour of James Prescott Joule.
A calories is another unit of energy. With its association with food, particularly for dieters, it is perhaps better known. In a science laboratory, a calorie is the energy needed to raise the temperature of one gram of water by one degree Celsius (at standard temperature and pressure). A warning to the dieters. Their calorie which they see on food boxes is actually a thousand ‘lab’ calories. For lab work, the following relation holds;
| Eq. 2 | 4.184 Joules = 1 calorie |
This is the relationship used in this website. But again, don’t get excited about this equation. You’ll quickly see that we are typically dealing with millions and millions of Joules.
Commonly, we relate energy to action. Often the action is considered to be work or heat. The following list relates energy to action.
Table 1: Energy Usage by the Joule
| Action | Amount (Joules) |
| Yearly solar emissions | 1034 |
| Exploding volcano | 1019 |
| Energy to launch space shuttle | 1013 |
| One litre of gasoline | 107 |
| Human’s daily energy needs | 107 |
| Candy bar | 106 |
| Burning match | 103 |
| Human heartbeat | 0.5 |
In the above, the amount of Joules is given by an exponent. Consider the exponent to be the number of zeros. For example the typical candy bar has 106 Joules or 1 000 000 Joules.
Matter
At one time we considered matter to be indestructible. That is, matter is neither created nor destroyed. Since then, we’ve learned about nuclear fission and fusion. Now we know that matter can be destroyed via fission as within a manmade nuclear reactor and created via fusion as within a star. But for this website we consider matter/energy to be a constant as shown in Equation 1 above. This is significant with regard to entropy.
Energy is finite and regulated. Our Sun fuses hydrogen atoms into the bigger helium atoms and thus sends forth the energy. We see it as light, feel it as heat and watch it encourage plants to grow. This is a good thing as we eat the grown plants or other animals which themselves eat plants. Our biological energy needs are completely and solely satisfied by what we eat.
Our civilization also needs energy. As we started down the path of technologically advanced civilizations, we simply burned wood for cooking food. Later we harnessed wind power using sails and directed water flow onto wheels to grind wheat into flour. Now, we’re splitting atoms to use the huge quantity of released energy to send electricity through our power grids.
But the most ubiquitous source of energy is the fossil fuels. Petroleum, natural gas and coal are the real energy provides for our civilization today. These fossil fuels power electrical generating stations, push airplanes through the skies, and, propel cars along the freeways. Yet, these energy sources aren’t limitless. They all started as plants about some hundreds of millions of years ago. These plants captured the Sun’s energy, just like plants of today. However, they weren’t able to release it. Thus the energy accumulated and through a variety of processes they were transformed. Given the hundreds of millions of years for this process, we can readily consider fossil fuels as non-renewable. Hence, we will eventually consume all fossil fuels available today.
Our Sun
The Sun is our energy source. Measurements in space have shown that a fairly steady energy flux from the Sun arrives at the top of our atmosphere. Similar measurements quantify the amount of energy that reaches the land surface. We can consider this energy to be renewable. This energy limit is all that is available for all living things on Earth.
Table 2: Estimated availability
| Energy flow | |
| From sun to top of atmosphere | 1387 Watts per square metre |
| To land surface | 1000 Watts per square metre, high noon |
| To land surface, average | 240 Watts per square metre, average over Earth |
| To land surface, in Joules | 1.127 x 1024 J/yr |
Note that a Watt is a rate of energy as in Joule per second.
Effectively all the renewable energy available to people comes from the Sun. This energy comes in a variety of shapes and forms. Because of it, the wind blows. Because of it, water evaporates from the oceans and falls onto the highlands so as to allow the water to flow back down to the oceans. Direct rays from the Sun power photosynthesis in plants. The Sun is necessary for (almost) all life on Earth.
The following table provides an estimate on the flow of energy through the Earth’s living things.
Table 3: Transfer to Living Things
| Totals | ||
| Solar power incident on earth, rate | 1 x 1017 | Watts |
| Solar power incident on earth, yearly total | 3.2 x 1024 | J/yr |
| Solar power incident on land | 2.2 x 1024 | J/yr |
| Maximum solar power taken by net primary producers on land | 2.2 x 1023 | J/yr |
| Maximum solar power taken by herbivores on land | 1.1 x 1022 | J/yr |
| Maximum solar power taken by carnivores | 5.5 x 1020 | J/yr |
| Global human energy consumption | 4 x 1020 | J/yr |
The above table quantifies the theoretical maximum energy available. It assumes a 5% efficiency rate in transferring energy from plants to animals and from animals into other animals. From this, in theory, humans are now capturing for themselves almost all the available energy that would be shared by all carnivores. But this energy consumption is used by human’s technology. Its relevance will become apparent when we consider the sustainability of our energy consumption.
While human technology does consume a lot of energy, most of it comes from fossil fuels rather than the existing biomass. The following table lists the various sources of our energy consumption.
Table 4: Energy Consumption
| Human Energy Consumption (2002) | ||
| Global human energy consumption | 4 x 1020 | J/yr |
| Primary energy consumption | 9405.0 | mtoe |
| Primary energy consumption | 3.95 x 1020 | J/yr |
| Primary energy consumption – oil | 1.48 x 1020 | J/yr |
| Primary energy consumption – natural gas | 0.96 x 1020 | J/yr |
| Primary energy consumption – coal | 1.0 x 1020 | J/yr |
| Primary energy consumption – nuclear | 0.26 x 1020 | J/yr |
| Primary energy consumption – hydro | 0.25 x 1020 | J/yr |
The units of mtoe are million tons of oil equivalent. It’s a huge amount of energy and is regularly used in annual summaries for national consumption. One mtoe has about 4 x 1016 joules of energy.
Note that the above value for the primary energy consumption doesn’t include biomass. That is, the amount of energy we derive from wood and other vegetation is so small that it isn’t worth including.
For comparison, we can estimate the energy needed to keep all the people alive. This is in the following table.
Table 5: People’s Biological Needs
| Minimum Energy Intake | |
| Average human male | 2500 kCalories/day |
| Average human male | 10.5 megajoules/day |
| Earth’s human population (2005) | 6 413 956 859 |
| Base living requirements | 2.452 x 1019J/yr |
From the above, we realize that while our biological energy needs are less than a tenth of our technological energy needs, it is still a huge amount of energy as noted in Table 1 above.
This is the crux of the challenge for civilization’s future. Is there enough energy for all of humanity’s ambitions? A lot of people live on the Earth’s surface. They all need to eat so as to live. Many want the comforts of an advanced civilization’s technology which requires lots of energy. The Earth is finite. Eventually we will reach a maximum number of people and a maximum energy consumption. And what sort of civilization will exist when these maximums or peaks are attained?
Entropy
Entropy is a simple concept with far reaching importance. Entropy tells us that energy, though indestructible, becomes less able. This is our experience on Earth and, except for black holes of outer space, seems to hold for everywhere in the universe. Less capable means that energy, once used, can’t do as much work or create as much heat.
Let’s picture this idea. Imagine a lake filled with water. At one end of the lake, the water empties through a river and descends over a waterfall. At the base of the waterfall is a mill. The mill uses the water to spin stones that grind the grain to make flour. After the water spins the stones, it flows to another lower lake. In this closed example, water is neither created nor destroyed. The water that starts at the top lake has the ability to do work, which it does when it descends through the waterfall and turns the wheel. The water at the bottom lake is the same to all appearances as the water in the top lake but it can’t do work. It’s ability has gone. That’s the picture.
Energy is like the water in this imaginary scenario. It exists. It starts with the ability to do work or perform an action which it naturally will do. Once it does so, it remains but its ability is gone. The entropy relates to where energy exists in this imaginary scenario. Energy that is akin to water in the top lake has the greatest amount of energy. Energy that is akin to water in the bottom lake has the least amount of energy. And, like water being unable to flow uphill, entropy of a system will not naturally improve.
What does entropy mean for us on Earth? Well, consider our solar system as a closed system. It has a given entropy. This entropy will naturally increase (capability decrease). Though there may be localized areas of the solar system where entropy decreases, on the whole it increases. The same is true for any system on Earth. Small locations may see drops in entropy especially with man-made works such as electrical generating station. But expand the boundaries of these small locations for the system to include the complete thermal cycle and the natural tendency to increase entropy remains.
The implication of entropy for humanity is that energy capability is naturally continuing to decrease. As we burn wood in furnaces, capture photons with photo-voltaic cells or split atoms in reactors, we make a brief local increase in energy ability which we use. But overall we increase entropy. In effect, our action increases the size of the topmost river in our imaginary scenario. Our actions allow more water to descend over the falls and driver more mills. But this means the water more quickly loses its capability. And we know it never recovers this.
This one-way nature of energy should be a warning to humanity. There is lots of energy about us but its entropy is continually increasing. Would a wise person be sure to make the best utility of the available energy? Do we make the best choices? Take a look at the consumption patterns to see where we are using energy today.
One question may still perplex you. Where does energy’s ability go to? Simply, it dissipates as heat. Cold areas get warmer. Cold areas don’t naturally get colder. As heat energy radiates out from stars or planets, the energy transfers to the atoms spread throughout the universe. If this were the only force, eventually all material objects of the universe will all have the same temperature. But that temperature would still be very darn cold so don’t expect a tropical paradise.

The Sun, in Detail
Our Sun is one big fusion reactor. Hydrogen atoms fling about throughout its volume. They sometimes hit each other, fuse, and, make Helium. Helium makes Oxygen. Oxygen fuses into Carbon. When two get together to make a third, the third has a little bit less mass. This mass loss appears as electromagnetic energy. The energy spreads somewhat unevenly across the infinite spectrum. But, the majority of it exists as visible light.
Sunshine to Earth
The energy emitted by our Sun travels in a straight line (mostly) until it hits something. Maybe it goes beyond our solar system, beyond our galaxy and to another galaxy where it strikes the retina of an alien species. But we won’t consider that on this website.
As our Sun is so much closer than other stars then much more light energy reaches us. This energy, known as the solar wind, acts very much like the wind that flows at the surface of the Earth. At the top of Earth’s atmosphere, the Sun delivers 342 Watts per square metre, non stop, and with very little variation.
The Magnetosphere
Next to nothing inhibits the Sun’s energy from reaching the top of Earth’s atmosphere. But, once the solar wind comes into the influence of the Earth, things begin happening. First, the magnetosphere directs some energy and particles away as shown in the following.

Though the magnetosphere greatly decreases the energy in the solar wind, some continues on toward Earth. But on encountering the Earth’s atmosphere, again more energy leaks out of the wind. The following gives an idea of the average energy disbursement.

While the above figure appears simplistic, it hides an amazingly complex energy flow. For example, if too much energy gets reflected the Earth could become a snowball again. If too much energy gets absorbed then the Earth becomes a heat-trap like the planet Venus. We’ll learn more about this when discussing climate change later.
Insolation
No matter where we stand on Earth during the day, we always say that the Sun is up in the sky. Just look up and our great yellow star will be shining down. But, the amount of annual light or light energy is different for every different location on Earth. Locations on the equator are closer to the Sun and thus will have great values. Locations at either poles will have much smaller values as the Sun is never directly overhead and, for weeks at a time, can be completely absent.
For the United States, the following shows the average annual solar radiation at ground level in kWh/m2/day.
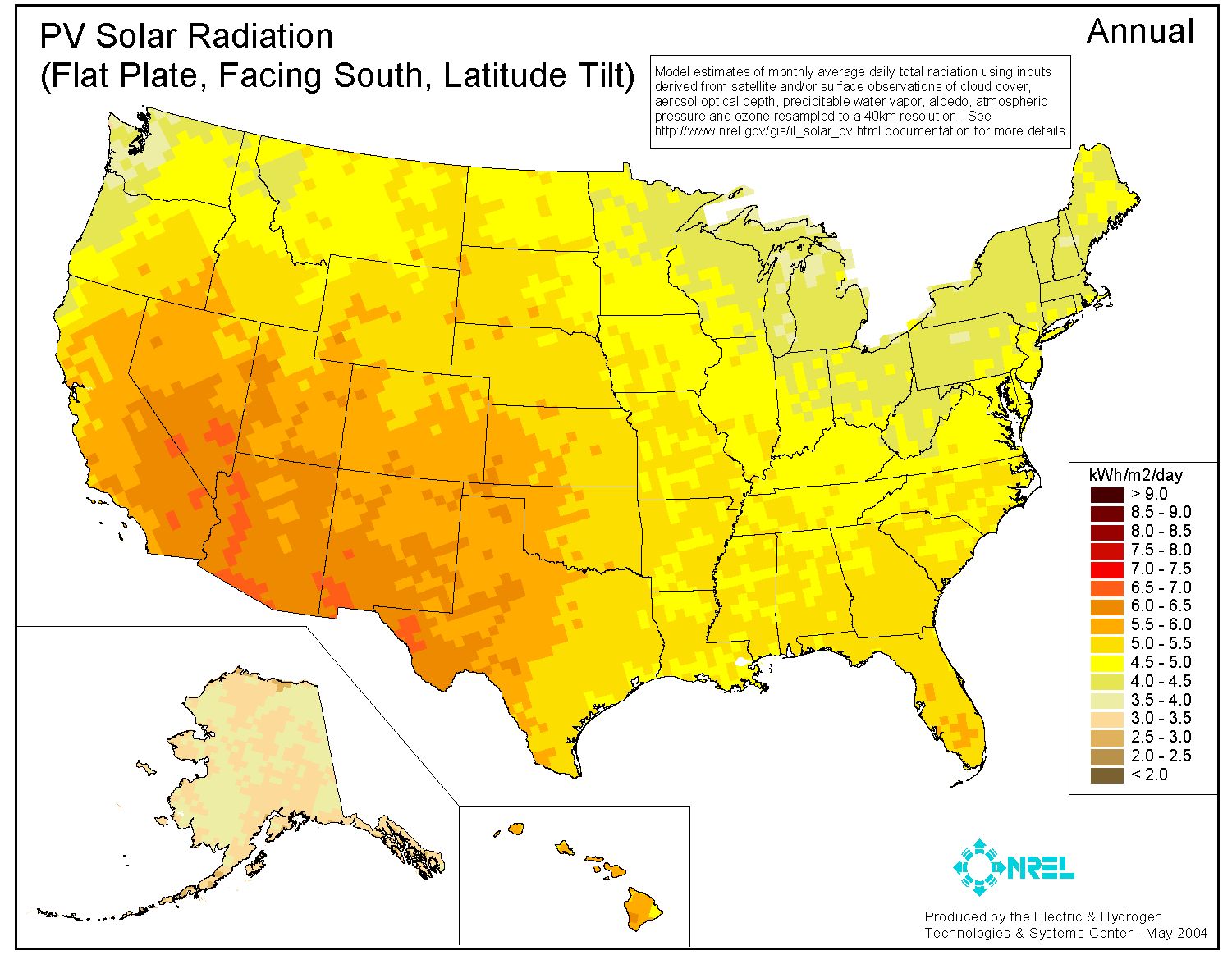
As to be expected, Alaska being farther from the equator, has low values of about 3 kWh/m2/day (avg of 11MW/day). However, places along the country’s southern border which are also at high altitudes have the highest annual radiation of about 7 kWh/m2/day (avg of 25 MW/day).
These solar radiation values are good references when planning a civilization’s future that includes renewable energy sources.
Power Generation
While the Sun’s energy does strike the Earth’s surface, humans still need to turn that energy into something that our technology can use. We let plants and their photosynthesis convert the Sun’s energy into power for our bodies. Let’s look into how we can do this for our mechanical devices.
We can’t eat solar energy but we can collect it and have it do our bidding. Many different ways exist to do this. But, the largest solar powered electrical uses the well known process of having the energy from the Sun heat a fluid. The heat is transferred to water which becomes steam. The steam enters a turbine which in response rotates. The rotation generates the electricity as shown in the following figure.

Though the Sun can feel hot, its radiant energy does not have the ability to efficiently drive a steam generator. Instead, we use mirrors to focus the Sun’s energy onto a fluid, in this case oil, and the heated oil supplies the energy to heat the water. This is the technique used at a solar power electrical generating station in the Mojave desert of the Unites States as seen in the following.
 |
 |
| from ReflecTech | from Sandia |
The following table has the value of the captured energy at the SEGS Location in the Mojave Desert Area in gross annual solar production of electricity (MWh);
| Plant ID# | Name | MWh |
| I | Daggett | 16500 |
| II | Daggett | 32500 |
| III | Kramer Junction | 68555 |
| IV | Kramer Junction | 68278 |
| V | Kramer Junction | 72879 |
| VI | Kramer Junction | 67758 |
| VII | Kramer Junction | 65048 |
| VIII | Harper Lake | 137990 |
| IX | Harper Lake | tbd |
| Total | 654544 |
In the above table, the gross annual solar is the four year average from 1998 to 2000 (see here).
The nine solar collection sites produce an annual 654544 MWh from fields covering 890 hectares. Or, the sunshine on the ground annually provides 2715 kWh/m2 of which we capture 73 kWh/m2, about a 2.7% efficiency. Afterward, some electricity is lost through conversion, delivery and the final consumption by us, the consumer. Hence, we will never realize the full potential of the Sun but we are still benefitting.
Sun Summary
The Sun provides 342 watts /m2 or 1.1e10 joules/m2 per year at the top of the atmosphere. But, the atmosphere greatly affects the solar wind so only a small fraction reaches the Earth’s surface. As well, the Earth spins so only part of it receives solar energy at any given time. The better locations on Earth’s land surface have an insolation of 7 kWh/m2/day or on average 25.2e7 Joules/m2/day or about 9e9 Joules/m2 annually. Using today’s advanced technology, we can capture the Sun’s energy and generate about 73 kWh/m2 annually. A typical refrigerator uses over 700 kWh per year. The people of the United States consumed over 3e13 kWh of electricity in the year 2007. The United States would need 4.2e7 hectares of equivalent solar collectors if the Sun was the primary source for all their power needs. This area is more than half the area of the complete state of Texas. Is this how we will allocate land usage for our future civilization?
Energy Values
Energy is neither created nor destroyed. Rather, it loses its potential to do work. A wood burning fire is an example. Fissioning an atom’s nucleus is another. Energy loss is usually associated with the outflow of heat. We use heat to power our technology. We can assess the value of an energy transfer method by looking at the ratio of the input energy to the output energy. The following table shows some ratios.
| Source | R3 Energy Ratio.
(output/input) |
Input % of lifetime output |
||
| Hydro | Uchiyama 1996 | 50 | 2.0 | |
|---|---|---|---|---|
| Held et al 1977 | 43 | 2.3 | ||
| Quebec | Gagnon et al 2002 | 205 | 0.5 | |
| Nuclear (centrifuge enrichment) | see table 1. | 59 | 1.7 | |
| PWR/BWR | Kivisto 2000 | 59 | 1.7 | |
| PWR | Inst. Policy Science 1977* | 46 | 2.2 | |
| BWR | Inst. Policy Science 1977* | 43 | 2.3 | |
| BWR | Uchiyama et al 1991* | 47 | 2.1 | |
| Nuclear (diffusion enrichment) | see table 1. | 21 | 4.8 | |
| PWR/ BWR | Held et al 1977 | 20 | 5.0 | |
| PWR/BWR | Kivisto 2000 | 17 | 5.8 | |
| Uchiyama 1996 | 24 | 4.2 | ||
| PWR | Oak Ridge Assoc.Univ. 1976* | 15.4 | 6.5 | |
| BWR | Oak Ridge Assoc.Univ. 1976* | 16.4 | 6.1 | |
| BWR | Uchiyama et al 1991* | 10.5 | 9.5 | |
| Coal | Kivisto 2000 | 29 | 3.5 | |
| Uchiyama 1996 | 17 | 5.9 | ||
| Uchiyama et al 1991* | 16.8 | 6.0 | ||
| unscrubbed | Gagnon et al 2002 | 7 | 14 | |
| Kivisto 2000 | 34 | 2.9 | ||
| Natural gas | – piped | Kivisto 2000 | 26 | 3.8 |
| Natural gas | – piped 2000 km | Gagnon et al 2002 | 5 | 20 |
| LNG | Uchiyama et al 1991* | 5.6 | 17.9 | |
| LNG (57% capacity factor) | Uchiyama 1996 | 6 | 16.7 | |
| Solar | Held et al 1997 | 10.6 | 9.4 | |
| Solar PV | rooftop | Alsema 2003 | 12-10 | 8-10 |
| ground | Alsema 2003 | 7.5 | 13 | |
| amorphous silicon | Kivisto 2000 | 3.7 | 27 | |
| Wind | Resource Research Inst.1983* | 12 | 8.3 | |
| Uchiyama 1996 | 6 | 16.7 | ||
| Kivisto 2000 | 34 | 2.9 | ||
| Gagnon et al 2002 | 80 | 1.3 | ||
| Aust Wind Energy Assn 2004 | 50 | 2.0 |
* In IAEA 1994, TecDoc 753.
Source is here.
All the ratios in the above come with assumptions and considerations. Compare the above to the following table’s values and you can see that generating these values isn’t easy.
| Process | Energy Profit Ratio | |
| Nonrenewable | Oil and gas (domestic wellhead, 1940s) | Discoveries > 100 |
|---|---|---|
| Oil and gas (domestic wellhead, 1970s) | Production 23 | |
| Coal(mine mouth) 1950s | 80 | |
| Coal(mine mouth) 1970s | 30 | |
| Oil shale | 0.7 to 13.3 | |
| Coal liquefication | 0.5 to 8.2 | |
| Geopressured gas | 1 to 5 | |
| Renewable | Ethanol (sugarcane) | 0.8 to 1.7 |
| Methanol (wood) | 2.6 | |
| Solar space heat, flat-plate collector | 1.9 | |
| Electricity Production | Coal, US averagetd> | 9 |
| Hydropower | 11.2 | |
| Nuclear (light-water reactor) | 4.0 | |
| Solar, power tower | 4.2 |
from, Heinberg, “The Party’s Over” .
However, these ratios only consider the application of energy by humans to obtain energy for humans. Precious little consideration or value gets considered for any loss of energy capture by autotrophs. A mine lays waste thousands of hectares and may take hundreds to thousands of years to return to a pristine state. The ratios don’t include this consideration. But these ratios will be very useful for mapping a sustainable future for civilization.
Energy Content
The following provides some typical values for energy content. These will also be useful in mapping a future for civilization.
Food
From USDA Home and Garden Bulletin Number 72.
| Food | Mass(g) | Energy(MJ) | Density(kJ/g) |
|---|---|---|---|
| Cheddar Cheese | 28 | 0.477 | 17 |
| Milk 2% | 244 | 0.507 | 2.07 |
| Halibut | 85 | 0.498 | 5.86 |
| Rice, wild | 164 | 0.694 | 4.24 |
| Apple | 138 | 0.339 | 2.46 |
Needs
Human’s Annual Energy Needs, with drastic assumptions about activity levels.
| Human Time of Life | Joules x109 |
|---|---|
| Infant | 1.1456 |
| Child | 2.75 |
| Male | 3.36 |
| Female | 3.05 |
References
- USDA, “Nutritive Value of Foods“,2002.
- DOE and ORNL, “Biomass Energy Databook“,2006.
Fuel
From ORNL, these sources of energy are physical heat rather than edible food. These liquid fuels assume standard temperature and pressure.
| Fuel | EnergyDensity | Units |
|---|---|---|
| Ethanol HHV | 23.4 | MJ/litre |
| Propane | 25.5 | MJ/litre |
| Automotive Gasoline | 34.8 | MJ/litre |
| Jet Fuel (naptha) | 35.5 | MJ/litre |
| Crude Petroleum | 38.5 | MJ/litre |
| Diesel Motor Fuel | 38.7 | MJ/litre |
In the above, an MG is a megajoule or 106 Joules.
We can also use natural solid material to provide (heat) energy. The following values also come from ORNL.
| Fuel | EnergyDensity | Units |
|---|---|---|
| Switchgrass | 17.1 | MJ/kilogram |
| Wood HHV, bone dry | 22 | MJ/kilogram |
| Coal Anthracite | 25.2 | MJ/kilogram |
where HHV higher heating value
Activities
In a society grounded upon electricity, we’ve come to rely upon its utility. Even if wonderfully optimized, we use lots. The following associates a common household activity with its energy consumption.
| Activity | Energy (kWh) | Joules |
|---|---|---|
| Clothes Dryer (1 load) | 1.89 | 6792453 |
| Clothes Washer (1 load/hot wash) | 6.42 | 23094000 |
| Clothes Washer (1 load/cold wash) | 0.94 | 3396226 |
| Electric Stove (1 family meal) | 4.15 | 14943396 |
| Dishwasher (1 load) | 3.02 | 10867924 |
| AC Central 20 degrees (1 hour) | 2.64 | 9509433 |
References
Embodied Energy
Objects can release energy via chemical reactions as with fire. Usually, humans put much more energy into fabricating a product than what can ever come out. This emplaced energy or embodied energy, as seen in the following table, gives an indication of this great disparity.
| Embodied Energy | ||
| MJ/kg | MJ/m | |
| Aggregate | 0.10 | 150 |
| Stone (local) | 0.79 | 2030 |
| 0.94 | 2350 | |
| Concrete (30Mpa) | 1.30 | 3180 |
| Concrete precast | 2.00 | 2780 |
| Brick | 2.50 | 5170 |
| Cellulose insulation | 3.30 | 112 |
| Steel (recycled) | 8.90 | 37210 |
| Steel | 32.00 | 251200 |
| Plywood | 10.40 | 5720 |
| Glass | 15.90 | 37550 |
| Fibreglass insulation | 30.30 | 970 |
| Zinc | 51.00 | 371280 |
| Brass | 62.00 | 519560 |
| PVC | 70.00 | 93620 |
| Copper | 70.60 | 631164 |
| Paint | 93.30 | 117500 |
| Linoleum | 116.00 | 150930 |
| Polystyrene Insulation | 117.00 | 3770 |
| Carpet (synthetic) | 148.00 | 84900 |
| Aluminium (recycled) | 8.10 | 21870 |
| Aluminium | 227.00 | 515700 |
We’ll use these values when we establish energy accounting principles for a future civilization.
Now that you have a solid understanding of energy, advance to our page on energy and the ecosystem.
